|
Enhancement of electro-optic activity by introduction of a benzyloxy group to conventional donor-π-acceptor molecules Xianqing Piao, Xianming Zhang, Shinichiro Inoue, Shiyoshi Yokoyama, Isao Aoki, Hideki Miki, In this work, we investigated the enhancement of the electro-optic response by introducing benzyloxy group as an additional donor part on conventional donor-π-acceptor molecules. This new type donor exhibited a strong solvatochromic effect, indicating an extra donation to the π-conjugated bridge, which shifted the charge-transfer absorption of the chromophores to the lower energy region. Furthermore, the simple modification on the donor moiety resulted in about 2-fold improvement in the first hyperpolarizability and macroscopic electro-optic coefficient (r33) over the benchmark 2-dicyanomethylen-3-cyano-4-{2-[E-(4-N,N-di(2-acetoxyethyl)-amino)-phenylene-(3,4-dibutyl)thien-5]-E-vinyl}-5,5-dimethyl-2,5-dihydrofuran (FTC) counterparts. An r33 above 100 pm/V is ultimately achieved in conventional guest−host system. Graphical abstract A series of nonlinear optical chromophores with modified electron donor unit has been synthesized and their application as electro-optic materials has been investigated. Compared with the nosubstituted analogue, novel chromophores achieve higher electro-optic coefficients. 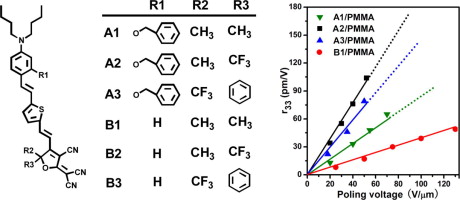
doi:10.1016/j.orgel.2011.03.010 |