|
Infrared Spectroscopy of Ionic Liquids Consisting of Imidazolium Cations with Different Alkyl Chain Lengths and Various Halogen or Molecular Anions with and without a Small Amount of Water Toshiki Yamada, Yukihiro Tominari, Shukichi Tanaka, Maya Mizuno Infrared spectroscopy was performed on ionic liquids (ILs) that had imidazolium cations with different alkyl chain lengths and various halogen or molecular anions with and without a small amount of water. The molar concentration normalized absorbance due to +C−H vibrational modes in the range of 3000 to 3200 cm−1 was nearly identical for ILs that had imidazolium cations with different alkyl chain lengths and the same anions. A close correlation was found between the red-shifted +C−H vibrational modes, the chemical shift of +C(2)−H proton, and the energy stabilization of the hydrogen-bonding interaction. The vibrational modes of the water molecules interacting with anions in the range between 3300 and 3800 cm−1 was examined. The correlation between the vibrational frequencies of water, the frequencies of +C−H vibrational modes, and the center frequency of intermolecular vibrational modes due to ion pairs was discussed. 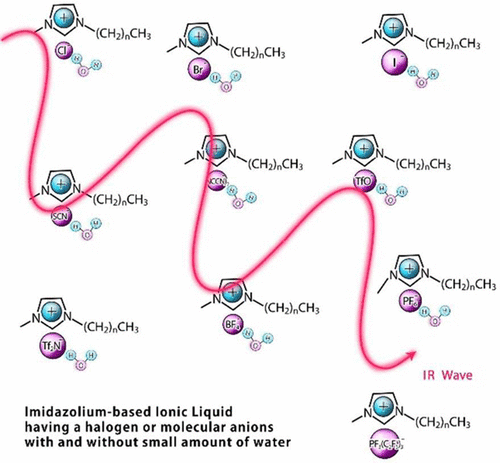 doi:10.1021/acs.jpcb.7b01429 |