Reserch
|
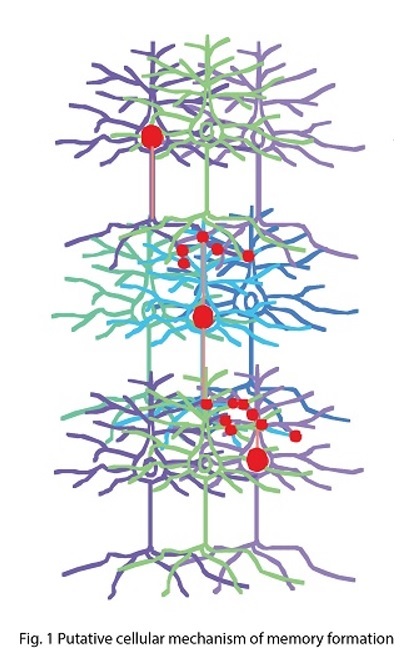
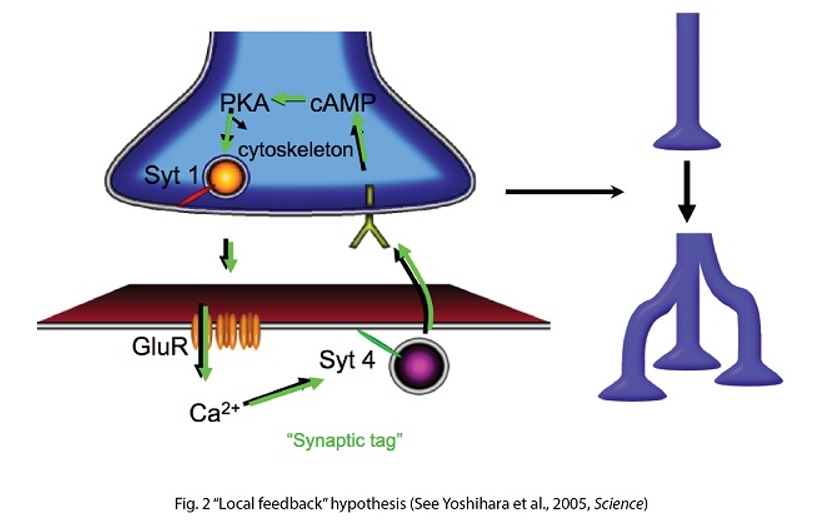
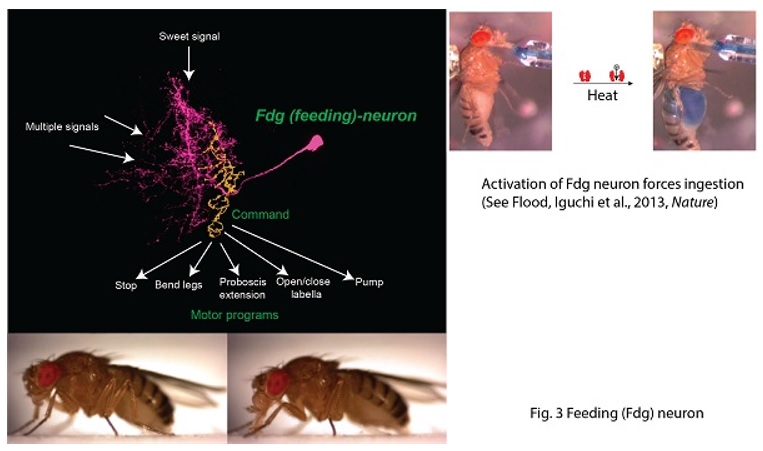
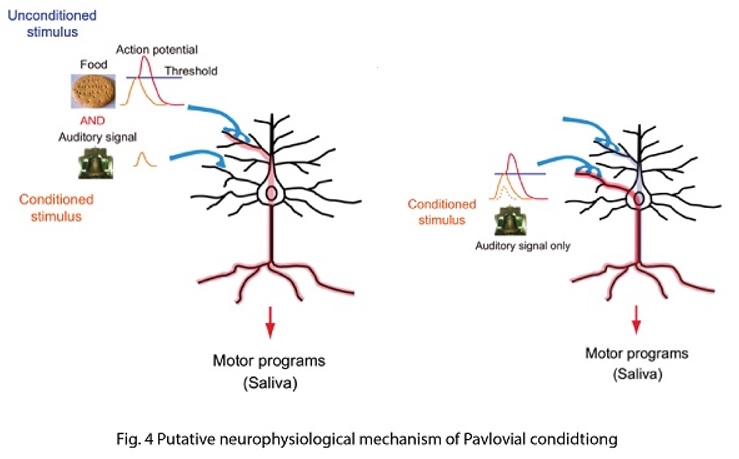
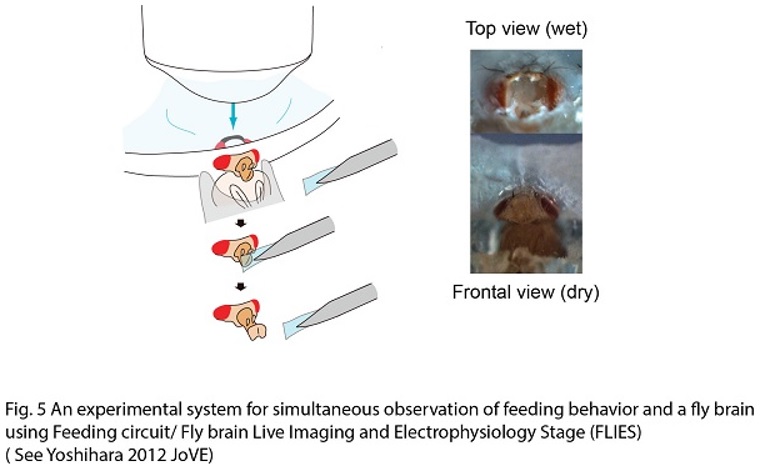
Connecting synaptic plasticity to memory using Drosophila feeding circuit and Drosophila embryonic neuromuscular junction. My lab is trying to ask a fundamental question “How do we remember?” by studying synaptic physiology in the fruit fly, Drosophila. I believe that memory is stored as a series of neurons connected through strengthened synapses, in which sequential firing of the neurons allows recall of specific events (Fig. 1). Therefore, understanding mechanisms of synaptic modification is a key to understanding mechanisms underlying memory. Taking advantage of a combination of synaptic physiological methods on highly plastic Drosophila embryonic neuromuscular synapses (neuromuscular junction, NMJ) and sophisticated Drosophila genetics, we have proposed a novel hypothesis, “local feedback model” as a potential molecular and cellular basis of memory formation (Yoshihara et al., 2005, Science 310: 858-863; Fig. 2). In this model, I postulate that mutual intensification between presynaptic and postsynaptic cells by a positive feedback loop at single synapses keeps individual synapses potentiated, leading to eventual morphological change and perpetually strengthened synapses, storing memory. In my lab, we are testing this working hypothesis to answer the question “how do we remember?”, by a novel approach using a pair of feeding command neurons, Feeding neuron (Fdg neuron; Fig. 3). We have performed behavioral screening on NP lines (Yoshihara and Ito, 2000, Drosoph. Inf. Serv., 83, 199-202) to establish a new system to connect synaptic plasticity to memory, and finally identified the Fdg neuron (Flood, Iguchi et al., 2013, Nature, 499: 83-87), which is ideal for neurophysiological analysis of classical conditioning demonstrated by Ivan Pavlov (Fig. 4) to connect synaptic plasticity to memory mechanism. We are now establishing novel protocols of Pavlovian conditioning to “witness” memory formation as real-time synaptic change in an experimental system I have devised for simultaneous observation of the brain and behavior of a single fruit fly (Yoshihara, 2012, JoVE; 62, 3625; Fig. 5). Through these novel approaches using Drosophila allowing us to perform comprehensive genetic analyses, we are trying to understand basic principle on molecular and cellular mechanism of memory formation. |
Acknowledgements
We greatly thank for generous support to Moto lab by Dr. Iwao Hosako, the Director General of the Advanced ICT Research Institute, Dr. Kazuhiro Oiwa, the Distinguished Researcher of the National Institute of Information and Communications Technology, and Dr, Hiroaki Kojima, the Director of Bio ICT Laboratory.